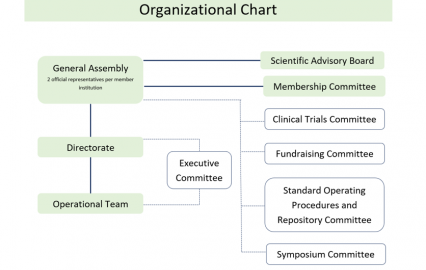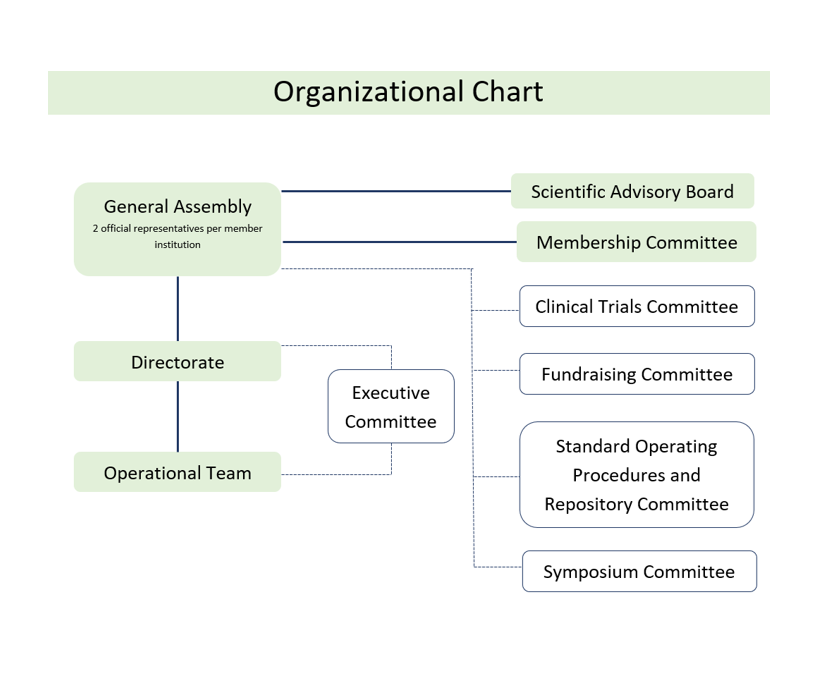

Organization
The WIN Consortium is organized in the following groups who collectively work together to achieve WIN's common goals
- General Assembly: Two representatives of each member institution are invited to participate in the general assemblies;
- Directorate: Maximum of 18 elected Directors;
- Operational Team: Administers the daily workings of the WIN Consortium;
- Executive Committee: constituted by Chair, Vice-Chair, Heads of special committees, Chief Medical Officer, Chief Operating and Scientific Officer, Director of Operational Team and special advisors, guide WIN’s operations;
- Scientific Advisory Board (SAB): Leading oncology experts provide strategic guidance and direction for our trials, research and scientific programs;
- Special committees: membership, clinical trials, symposium, fundraising, and standard operating procedures and repository.